Abstract
Introduction: Prognosis remains poor for most acute myeloid leukemia (AML) patients (pts). Enhanced risk-stratification, novel & individualized therapies to improve outcomes are needed. The RAS -pathway (NRAS, KRAS & HRAS& among others the regulator SHP-2 encoded by PTPN11) controls cell proliferation, cell differentiation & apoptosis & mutations in this pathway are common in many human malignancies, including AML. Previous studies of the prognostic impact of RAS mutations (mut) in AML demonstrated variable & context specific results. The prognostic impact in pts receiving allogeneic stem cell transplantation (HSCT) remains to be elucidated.
Patients & Methods: We analyzed the biologic & prognostic impact of RAS pathway mut(i.e. NRAS, KRAS, HRAS & PTPN11) in 113 AML pts (median age at HSCT 64 years [y]; range 32-75y) who received a non-myeloablative HSCT (Fludarabin 3x30mg/m^2 & 2Gy total body irradiation) at our institution. At diagnosis, cytogenetics & the mut status of CEBPA, NPM1 & presence of FLT3- ITD were assessed. We analyzed RAS pathway mut & the mut status of other AML-associated genes such as TET2, IDH1 & IDH2 on diagnostic bone marrow (BM) samples by targeted resequencing on a panel comprising 54 recurrently mutated (mut) genes in myeloid malignancies. For 104 pts receiving HSCT in complete remission (CR) or CR with incomplete recovery (CRi) outcome analysis was performed. Median follow up after HSCT was 6.0y.
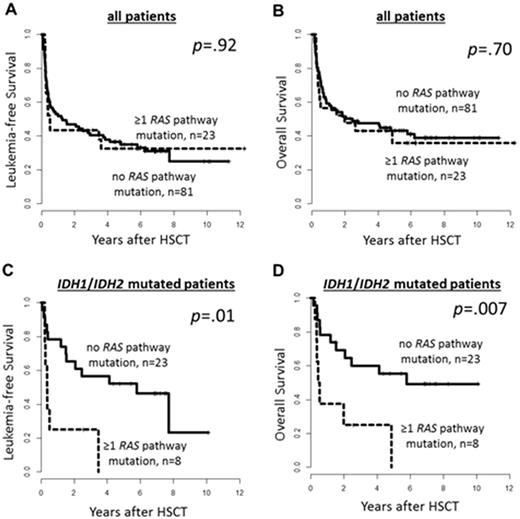
Results: In the whole cohort we found 23 AML pts (20%) with RAS pathway mut. Nineteen pts had NRAS, 6 pts PTPN11, 3 pts KRAS mut. None of the pts had a HRAS mut. Sixteen of these 23 AML pts had one RAS pathway mut (NRAS mut [n=13] & PTPN11 mut [n=3]), 6 had two RAS pathway mut (NRAS double mut [n=2], NRAS & KRAS mut [n=1], NRAS & PTPN11 mut [n=2], KRAS & PTPN11 mut [n=1]) & one pts had three RAS pathway mut (KRAS & double NRAS mut). 59% of NRAS mut (13/22) & 50% of PTPN11 mut (3/6) were sole RAS pathway mut. All 3 KRAS mutwere subclonal and accompanied other RAS pathway mut. RAS pathway mutpts were more likely to have de novo AML (p=.05) & were more frequently CEBPA mutated (p=.03). RAS pathway mut were mutually exclusive with TET2 mut (n=23, 27% in RAS pathway wild type pts vs . n=0, 0% in RAS pathway mut pts, p=.01). In the whole cohort no significant differences in leukemia-free (LFS) or overall survival (OS) for pts with or without RAS pathway mut (Figure 1A & B) was observed. However, when we restricted our analysis to IDH1 (n=15)& IDH2 (n=18) mut AML pts we observed a significant shorter LFS (p=.01) & OS (p=.007 , Figure 1C & 1D, respectively) for pts with RAS pathway mut, which may indicate an interaction of IDH1 / IDH2 & RAS pathway mut. RAS pathway mut were equally distributed between IDH mut & IDH wildtype pts (p=.44). Fifteen IDH1 mut pts harbored 5 RAS pathway mut (NRAS mut [n=1], NRAS double mut [n=1], NRAS & KRAS mut[n=1]) & 18 IDH2 mut patients harbored 5 RAS pathway mut (i.e. NRAS mut [n=4] & PTPN11 mut [n=1]). All RAS pathway mut were subclonal to the concomitant IDH mut. These findings suggest that RAS pathway mut may succeed the IDH mut, rendering the AML phenotype more aggressive by a yet to be identified mechanistical cooperation.
Conclusion: Our data revealed novel biological & clinical aspects of RAS pathway mut in AML. In our cohort, RAS pathway mut were mutually exclusive with TET2 mutations. In AML pts receiving HSCT no prognostic impact of RAS pathway mut was observed in the whole cohort. However, in pts with IDH1 or IDH2 mut subclonal RAS pathway mut associated with worse outcome in HSCT treated AML pts indicating a biological cooperation between theses genetic alterations. Especially, pts harboring these mut combinations may benefit from treatment with IDH1 & IDH2 and /or RAS pathway inhibitors.
Franke: Novartis: Honoraria, Research Funding; Pfizer: Honoraria; Takeda: Consultancy. Schwind: Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.